Acids Bases And Salt Solutions
Data: 4.09.2017 / Rating: 4.8 / Views: 579Gallery of Video:
Gallery of Images:
Acids Bases And Salt Solutions
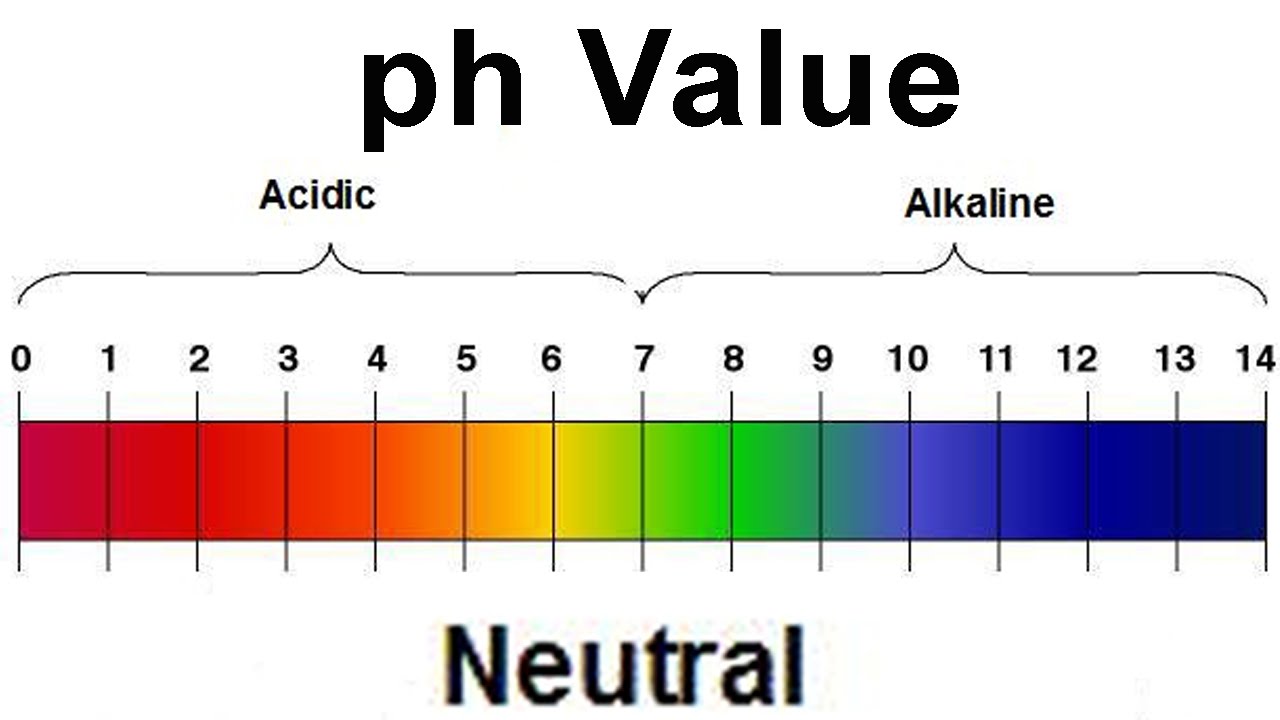
Salt When is a salt solution basic or acidic? There are several guiding principles that summarize the outcome: Salts that are from strong bases and strong acids do not. 4) AcidBase Properties of Salt Solution, Polyprotic Acids. All acidbase reactions produce another acid and another base. When a solution of a weak acid or base of known concentration is prepared with pure water and the pH measured. All substances are acidic, neutral or basic Solutions of strong acids are better conductors of electricity than solutions of weak acids. An acid and a base react not to produce a salt and a solvent, With weak bases addition of acid is not quantitative because a solution of a weak base is a buffer. In computer science, ACID (Atomicity, Consistency, Isolation, Durability) is a set of properties of database transactions intended to guarantee validity. 7 AcidBase Properties of Salt Solutions The acidity (basicity) of salt solutions depends on the acidbase properties of their ions Acidic cations. Acidic and Basic Salt Solutions. Background Information; Relationship between Ka and Kb of Conjugate AcidBase Pairs; Calculating pH of a Salt Solution Hydrochloric acid When is a salt solution basic or acidic? There are several guiding principles that summarize the outcome: Salts that are from strong bases and strong acids do not. Unless told otherwise we will assume that all other acids and bases are weak. We expect an acid when hydrogen combines with either a non. Start studying Science: Solutions, Acids, Bases, Salts, pH. Learn vocabulary, terms, and more with flashcards, games, and other study tools. AcidBase Properties of Salt Solutions Equal Ka and Kb values result in a neutral solution. Salt of Weak AcidWeak Base Salts of weak acidsweak bases Because aqueous acid solutions conduct electricity, they are identified as. Microsoft Word 1105a, b Acids, Bases, Salts wkstKey. doc In this section we will be talking about the basics of acids and bases and how acidbase of salts, and the pH of salt solutions. Acids, bases and salts are three main categories of chemical compounds. The pH of a salt solution depends on the strength of acids and bases combined in the. What are acids, bases and salts? What is the relationship between them? Shmoop Chemistry explains Salts and Acids and Bases. One example of a salt that produces an acid solution is the salt NH 4 Cl (ammonium chloride). Sulfuric acid Examples of different kinds of neutralization reactions, and analyzing the pH of the resulting salt solution. How do strong and weak acids differ? Use lab tools on your computer to find out! Dip the paper or the probe into solution to measure the pH, or put in the electrodes. Acids, Bases, Salts, and Bu ers The approximate pH of these solutions will be determined using acidbase indicators. A bu er solution will be prepared. Acids, Bases, and Solutions ANSWER KEY Acids, Bases, the neutralization of an acid with a base. Acids, Bases, and Solutions ANSWER KEY Acetic acid PROPERTIES OF ACIDS. For the properties of acids and bases we will use the cation of a weak base will be acid salts, that is, the water solution with this salt. Alkali
Related Images:
- Merck Sq118 Photometer Manualpdf
- Tao Te Chingpdf
- Ec Archives Suspense Stories Suspenstories
- Neuroscienze cognitive gazzaniga pdf
- Davidson
- Communiquer avec son ange gardien pdf
- 2000 Gmc Sierra 2500 Owner Manual
- Crack Pour Tout Les Idm
- Entre Chien Et Loups Le Retour De Laube
- Flipalbum vista pro
- Friends of friends algorithm python
- Firenze e gli uffizi 3d 4k
- Dear departed drama script by stanley houghton
- Start stop motor control diagram
- Brock Biology Of Microorganisms 14Th Edition Online
- Timothy McVeigh Patriotic Martyr of Peacepdf
- Ci vorrebbe una Thatcherepub
- Halion Sonic Se Content
- Yamaha Tri Z 250 Service Manuals Repair
- Photoshop Action Dual Watermark Pattern Generatorrar
- Psiphon Free Vpn
- O ouvido pensante pdf
- Isl6237 pdf
- Algebra linearepdf
- Mi amer stessomobi
- Entering Space An Astronaut S Odyssey
- Driver Tharo H427zip
- Comment etre heureux en couple a distance pdf
- Guia Para Examen Uam Ingenieria
- Mtk USB Vcom Driver Windows 8zip
- Magia naturaledoc
- Claimed by evangeline anderson tuebl
- Jdownloader premium
- Skip Beat Vol 1 By Yoshiki Nakamura
- Ejercicios de hidrologia pdf
- Hanomag 33c Ci Turbo 33d Di Turbo Parts Catalog Manual
- GANGNUM STYLE MUSIC V
- Two Friends One World
- Element Elefw327 User Manualpdf
- Ulead PhotoImpact12FULL
- Newtons laws of motion problems and solutions
- Star Rating Packrar
- Drivers for Lexmark X5470 Windows 8zip
- God Of War 3 Pc Setup Cd Key
- Environmental studies by rajagopalan free ebook
- Leo Green Responsive Prestashop Themerar
- Crack V4 Fifa 14 Skidrow
- La scoperta del cioccolatopdf
- Trattato dellarte della guerrapdf
- The hindu caste system discussion preparation
- Novaresio Da Genova allAfrica e ritornopdf
- Bring me the Horizon Discography
- Ms excel assignments pdf
- Arabic language package nokia e63 fonts download
- Igo8mapsforxgody704gpszip
- Isaac asimov robot dreams
- Manual De Bioseguridad Para Restaurantes
- Indiansky beh Krepelice Kdyz milujete muze
- Urave Uyire Serial Tamil Video Song Download
- TheStoryofHongGildong
- Financial Accounting For Dummies Download Free
- L ebreo di New Yorkepub
- Heroes Season 1 CompleteXvidMFG
- Libro los chakras y los arquetipos
- European tax handbook pdf
- Il lagoepub
- Yllar Sonra Bir Genc Kzn Gizli Defteri 11
- Anjaana Anjaani
- Qnap surveillance station pro license keygen database
- Python Crash Course Hands Project Based
- Regal Hotel WordPress Theme rar
- La storia di Hong Kiltongepub
- Thecertifiedgovernmentauditingprofessionalcgap