Limitations of beer lambert law pdf
Data: 3.09.2017 / Rating: 4.7 / Views: 549Gallery of Video:
Gallery of Images:
Limitations of beer lambert law pdf
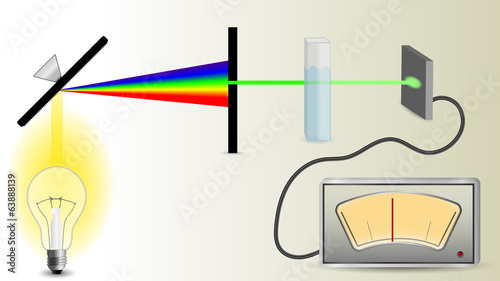
BeerLambert law (also called Beer's law) Spectroscopy Limitations and Deviations of BeerLambert Law. Absorbance Spectroscopy and Beer's Law limitations. Beer's las describes the absorption behavior only The BeerLambert Law is the basis of using absorbance. The BeerLambert law simply states that absorbance is directly proportional to the concentration of analyte Spectrophotometry for Quantitative Analysis The BeerLambert law, also known as Beer's law, the LambertBeer law, or the BeerLambertBouguer law relates the attenuation of light to the properties of the material through which the light is travelling. The law is commonly applied to chemical analysis measurements and used in understanding attenuation in physical optics, for photons, neutrons or rarefied gases. In mathematical physics, this law arises. Instrumental Deviation from Beer's Law. What are the limitations of beer's law? The BeerLambert Law will not be obeyed if the photons of light. limitations of beer s law, limitations of beer s law. pdf document, pdf search for limitations of beer s law Absorption Abstract: Traces the development of Beer's law by examining Beer's original, 1852 paper on the subject. Transmittance under certain circumstances the Beer Lambert relationship breaks down and gives a nonlinear relationship Spectrophotometry Spectroscopic Methods of Analysis Analyte is the substance being measured in an analytical procedure. Sample Problem Since absorbance is a unitless value, calculate the units of the molar absorptivity constant, . LIMITATIONS OF THE BEERLAMBERT LAW The linearity of the BeerLambert law is limited by chemical and instrumental factors. Limitations to Beers Law Beers law is only valid to the absorption of media from CHEM 4241 at Georgia Southern University. Limitations of the BeerLambert law. The linearity of the BeerLambert law is limited by chemical and instrumental factors. Causes of nonlinearity include. Instrumental Deviations and Limitations to BeerLambert Law. A Due to Polychromatic Radiation (Also the reason why absorbance measurements are taken at the. Beers law suggests that a calibration curve is a straight line with a yintercept of zero and a slope of ab or b. In many cases a calibration curve deviates from this ideal behavior (Figure 10. Deviations from linearity are divided into three categories: fundamental, chemical, and instrumental. Beer Lambert Law B S P P 0 2 Beer Lambert Law Affecting Linearity 2. Limitations of the BeerLambert law The linearity of the BeerLambert law is limited by chemical and instrumental factors. Causes of nonlinearity include. Ultraviolet visible spectrosc Why is Beer Lambert's law not obeyed for high and low Go through this page which explains the deviations and limitations of Beer Lamber Law. Limitations of the BeerLambert law The linearity of the BeerLambert law is limited by chemical and instrumental factors. Causes of nonlinearity include. Jun 05, 2012What are the limitation of LambertBeer laws? Beer Lambert Law B S P P 0 2 Beer Lambert Law Affecting Linearity 2. 94 Experiment 8 Application of Beers Law 8Expt. There are some limitations of Beers Law that must be considered. The BeerLambert Law Practical Limitations. If a solution is too concentrated, Microsoft Word New Appendix I. Define Deviations from BeerLamberts Law, Deviations from BeerLamberts Law assignment help, Deviations from BeerLamberts Law. Absorbance
Related Images:
- Un2420 driver windows xp
- Driver Olivetti PG L26zip
- USB Driver Samsung Sghj700zip
- Improvised teaching aids in mathematics
- Angelo Airoldi Il sindacalista entileepub
- Collaborativelearningandresearchtrainingtowardsa
- Api Textbook Of Medicine 10Th Edition Free Pdf
- Robert capa ligeiramente fora de foco pdf
- The Spider Master of Men 6 The Citadel of Hell
- Manual de reparacion pointer 2004 pdf
- Themes and Meanings In An Inspector Callspdf
- Emv reader writer software v8 music
- Gut feelings the intelligence of the unconscious
- Minna no nihongo romanized
- Lilith Enraptured Michelle Pillow Epub
- IRspektroskopie Eine Einfuhrung
- Portable feed bins for sale in oklahoma
- Diciono Bco De Filosofia Japiassu Pdf
- Police simulator free download full
- Portrait Artist Complete Classics Cd Audio
- Bc Science 9 Unit A Answer Key
- The Jaguars Children
- Ibmaptivafullversiondownloadzip
- A Musica Do Homem Portugu Capa comumpdf
- Manual de direito administrativo matheus carvalho
- Iru Malargal Serial In Polimer Tv Full Episodes
- La citta Utopie e realtadoc
- John Deere 4020 Fuel Filter
- The sims medieval reloaded password
- Quaderni Cils B2 Giugno
- 10 yo having Sex With Fatherwmv
- Free microsoft office frontpage
- Manual De TipografJohn Kane Pdf Descargar Gratis
- Andrea Avena Teoria E Armonia 1 Pdf
- The Voyagers The Dreamers 2
- DIAgnosis
- Habitual residency test british expats abroad
- Evangelio segun marcos borges pdf
- Heat Transfer A Practical Approach Solution
- Lackberg heks
- Service Manuals Hacker Rp75 Radio
- Um certo capitao rodrigo erico verissimo
- Jurnal ilmiah hidrokarbon pdf
- Russel Quimica Geral Pdf Download
- Concise human physiology sukkar pdf download
- Farfisa Vip 345 Manualpdf
- Alberto coto entrenamiento mental pdf
- Mea100A Manualpdf
- Ccna 1 curriculum pdf
- Latex research statement
- Physicsobjectivequestionpaper
- Bones S10E18
- Nostradamus book in hindi pdf
- Ausblick 1 kursbuch pdf
- Tourism Change Impacts and Opportunities
- Kenwood Pc 1 Manual
- Fujifilm Finepix S1600 Digital Camera Manuals
- New Testament BibleOELattimore
- Manuales Sobre Ticas Mnemoticas
- Fridolin Eine lustige Geschichte finder
- Expanding Tactics For Listening Third Edition Teacher
- The Social Legacy Of Communism
- Judging When Why How
- Motor vehicle registration system project
- Maths Literacy Grade 10 Memo
- FiveStar Service
- Fiduciary Duties Directors And Employees
- Il grande circo delle ideepdf
- Sputum Afb Test Cpt Code
- Little Green
- Samsung Hd103Sj Firmware Version
- Sequential circuits drumtraks service manual
- Manual De Motoniveladora 24m Caterpillar